
calibre
Visual Factory Calibre: The leading solution for industrial calibration management
Visual Factory Calibre ensures metrological compliance and MSA studies of your measuring equipment
What does visual factory calibre offer?
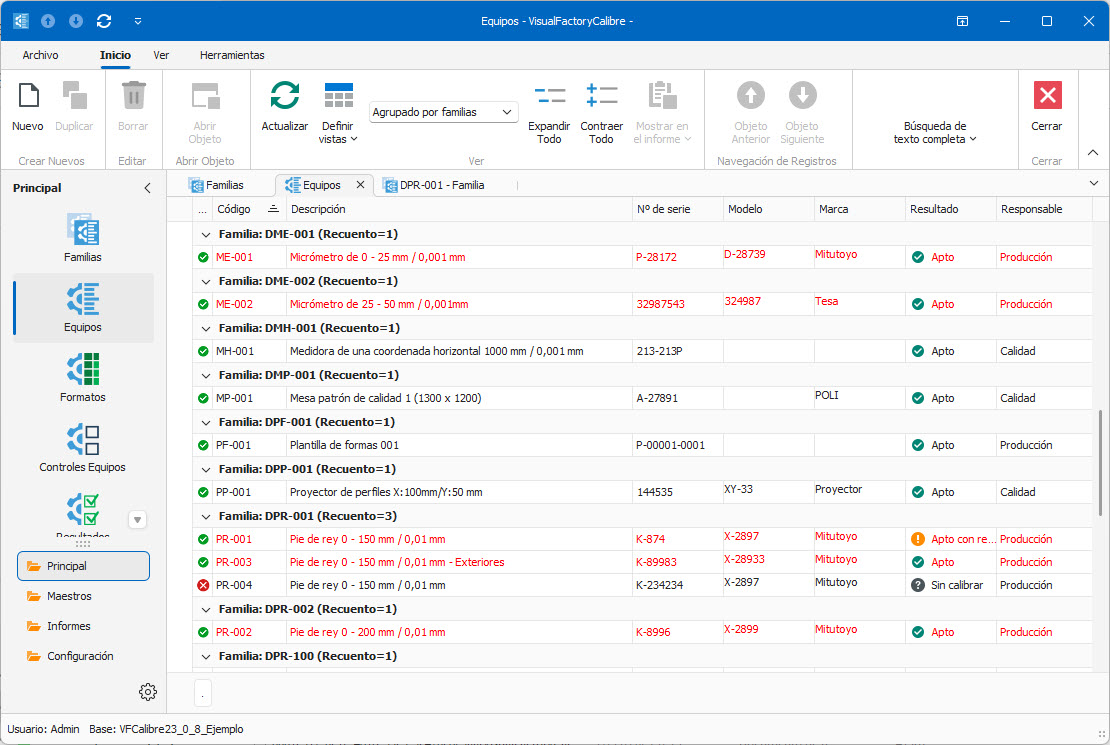
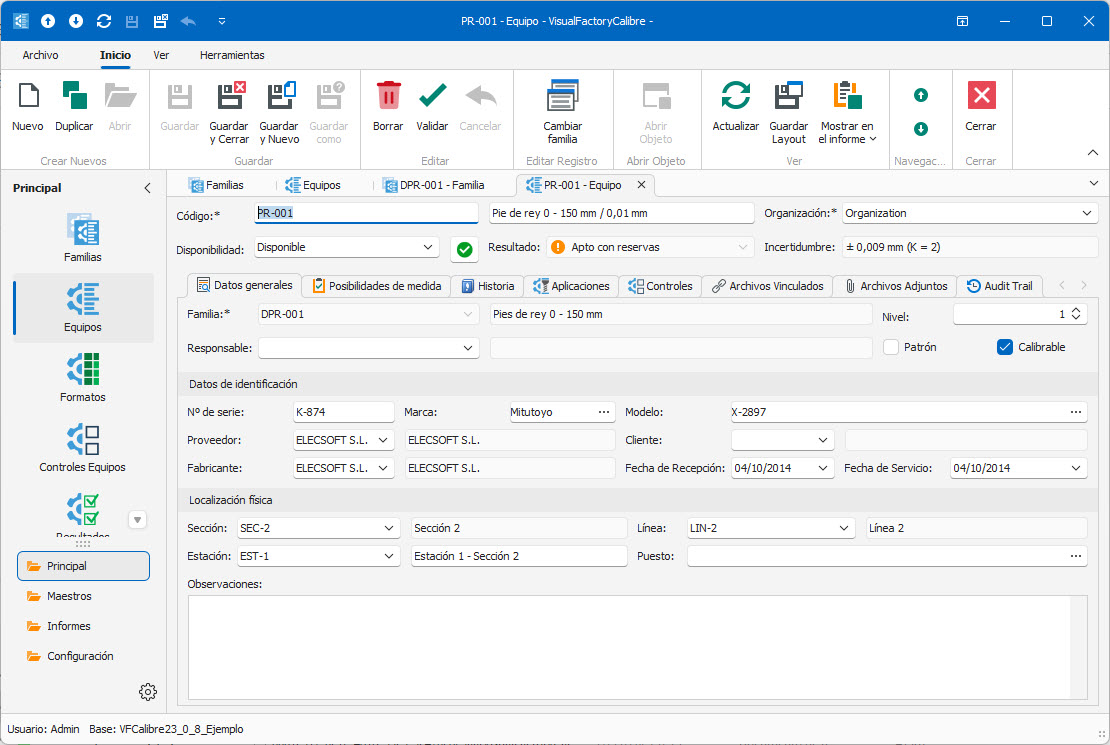
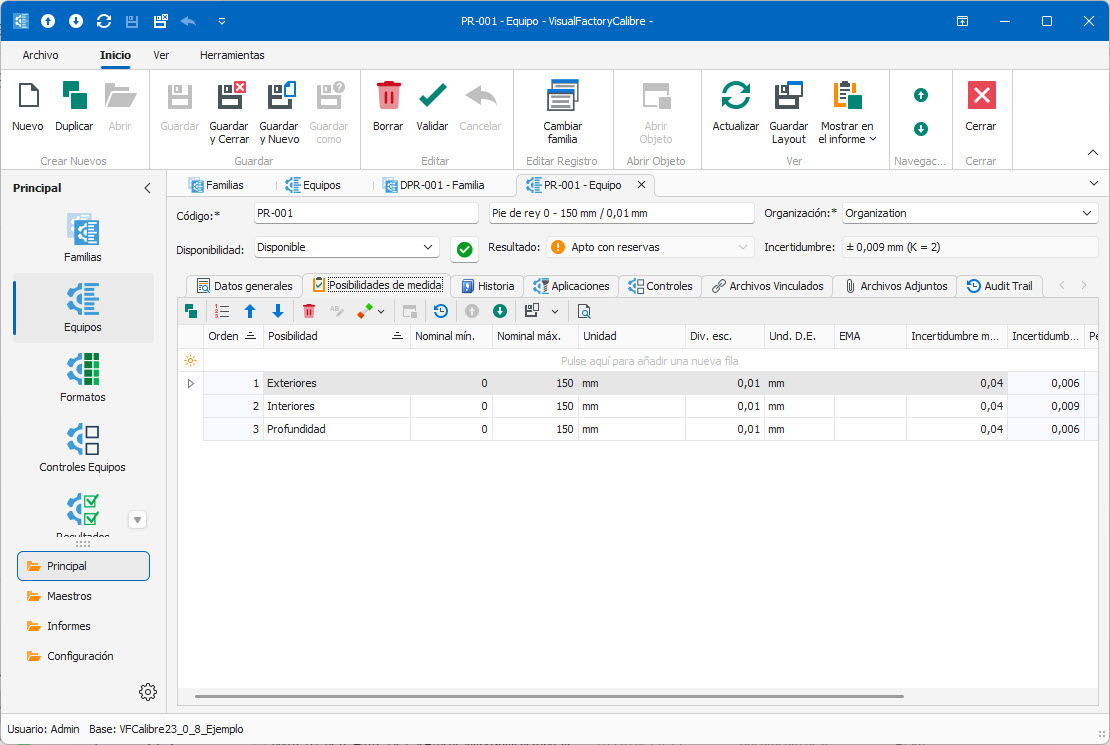
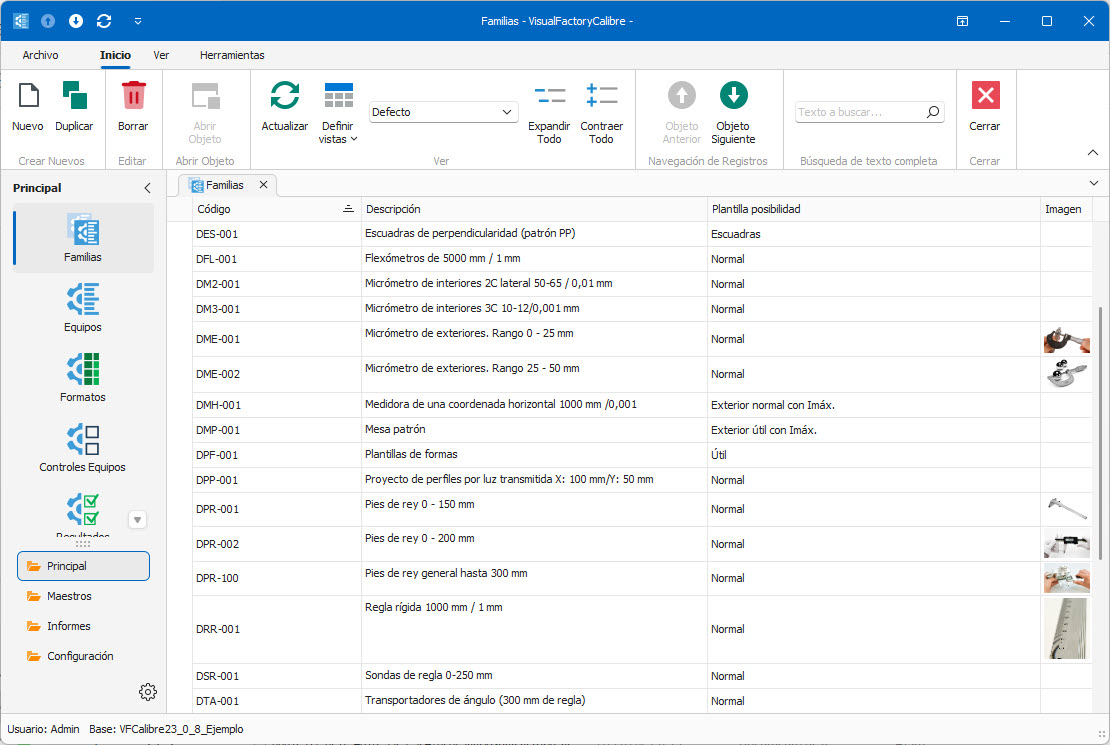
- Complete management of the inventory of measuring equipment.
- Grouping of equipment by family.
- Designed for any type of measuring equipment.
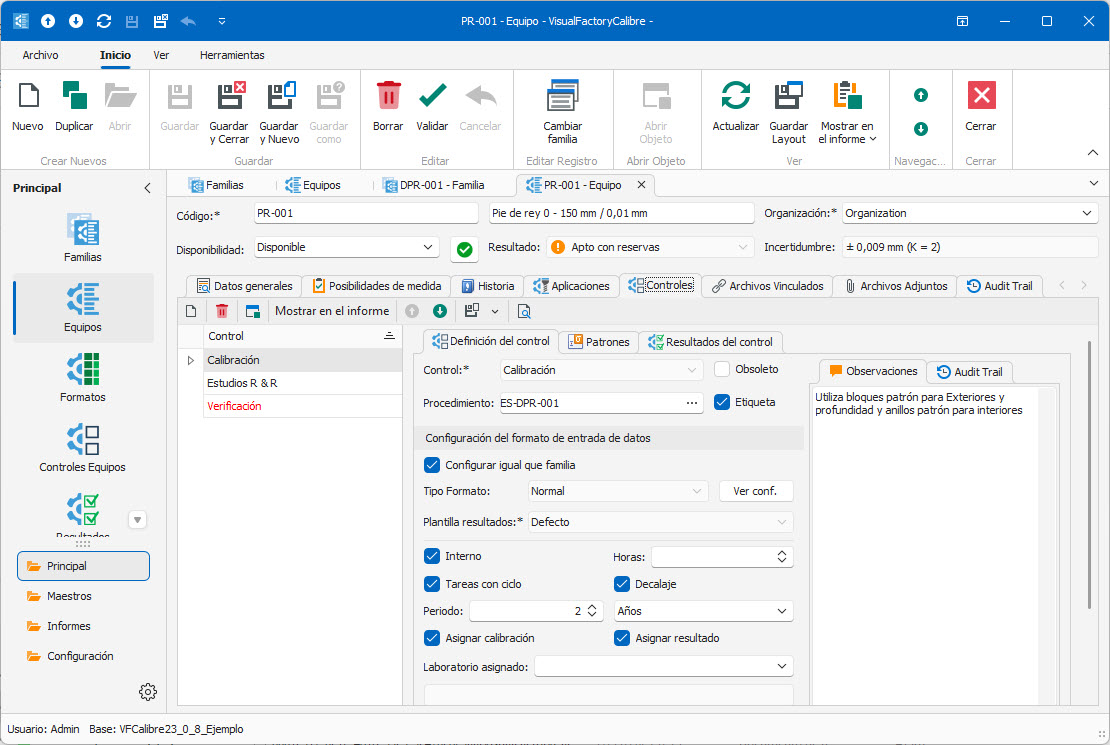
- In addition to calibration, multiple control processes can be defined: verification, MSA4, maintenance, …
- Wide range of ready-to-use calibration procedures.
- Configurable input formats for calibration and verification.
- For special calibration processes, full Excel integration is included.
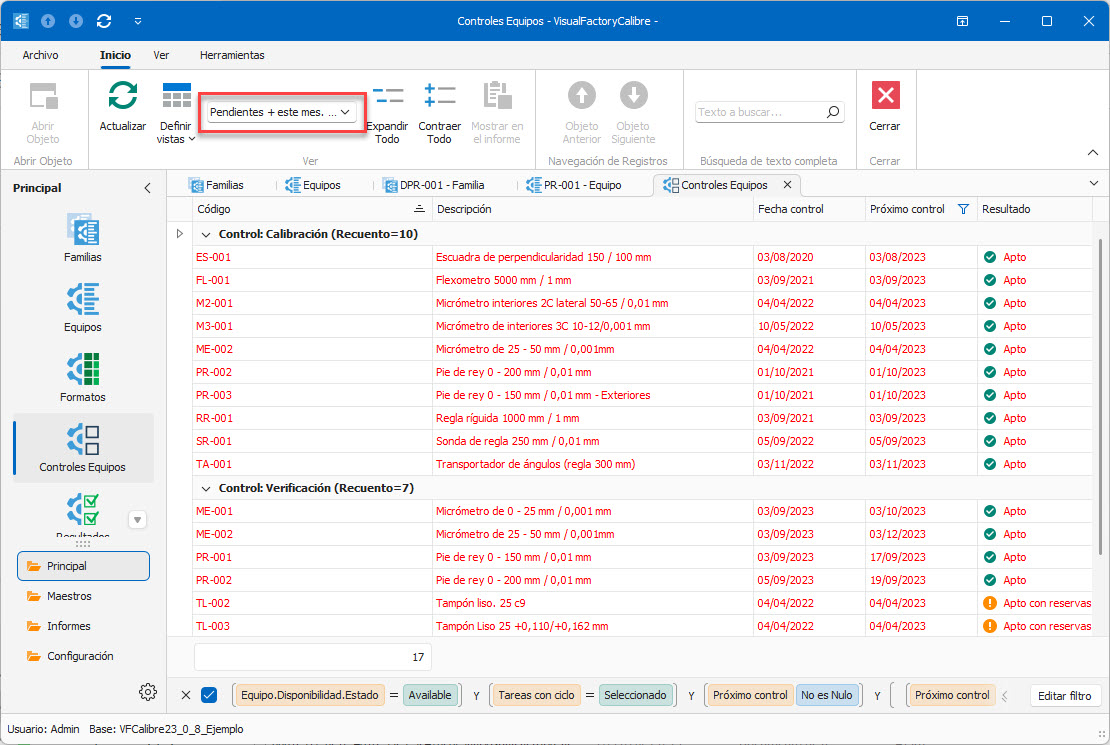
- Scheduled and unscheduled checking of equipment.
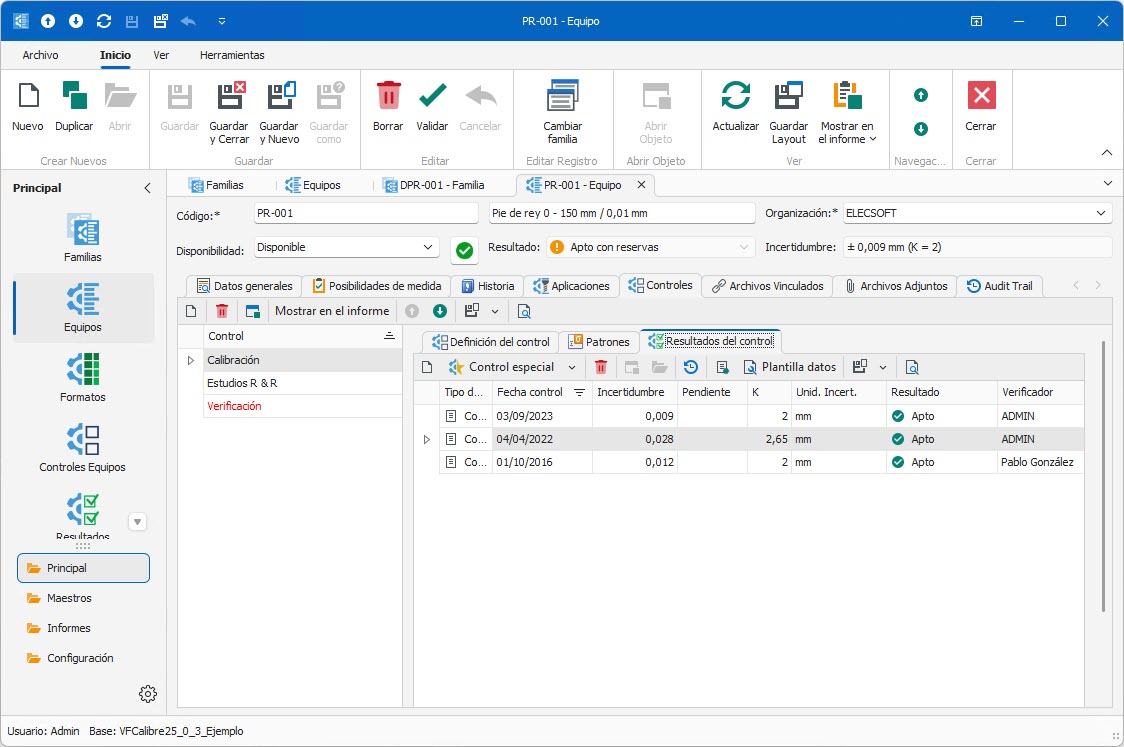
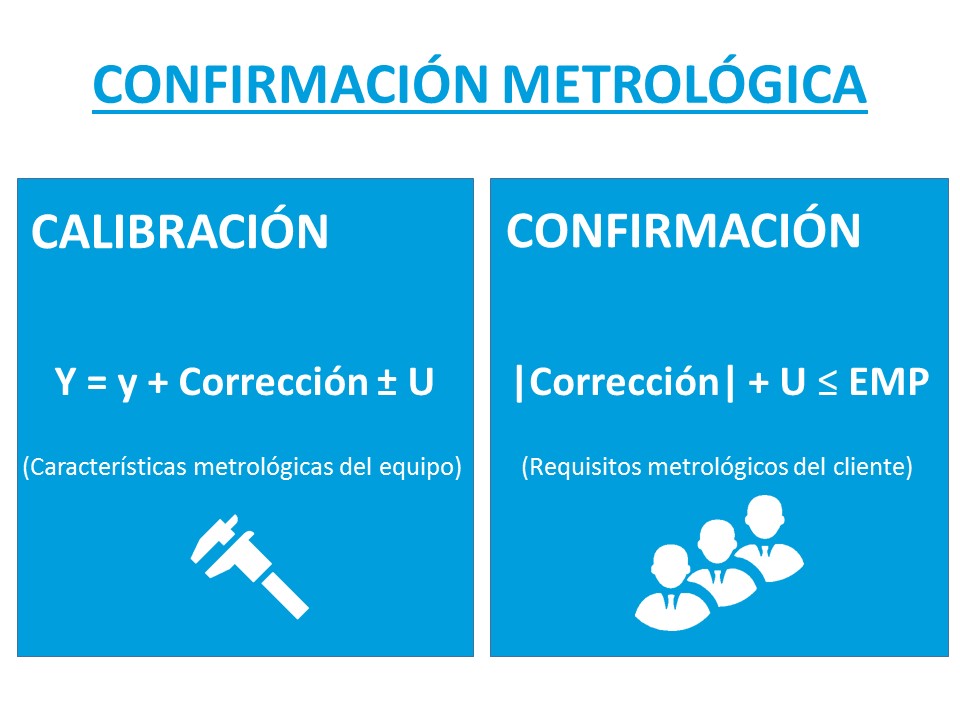
- Ensures traceability of results.
- Calibration target tracking.
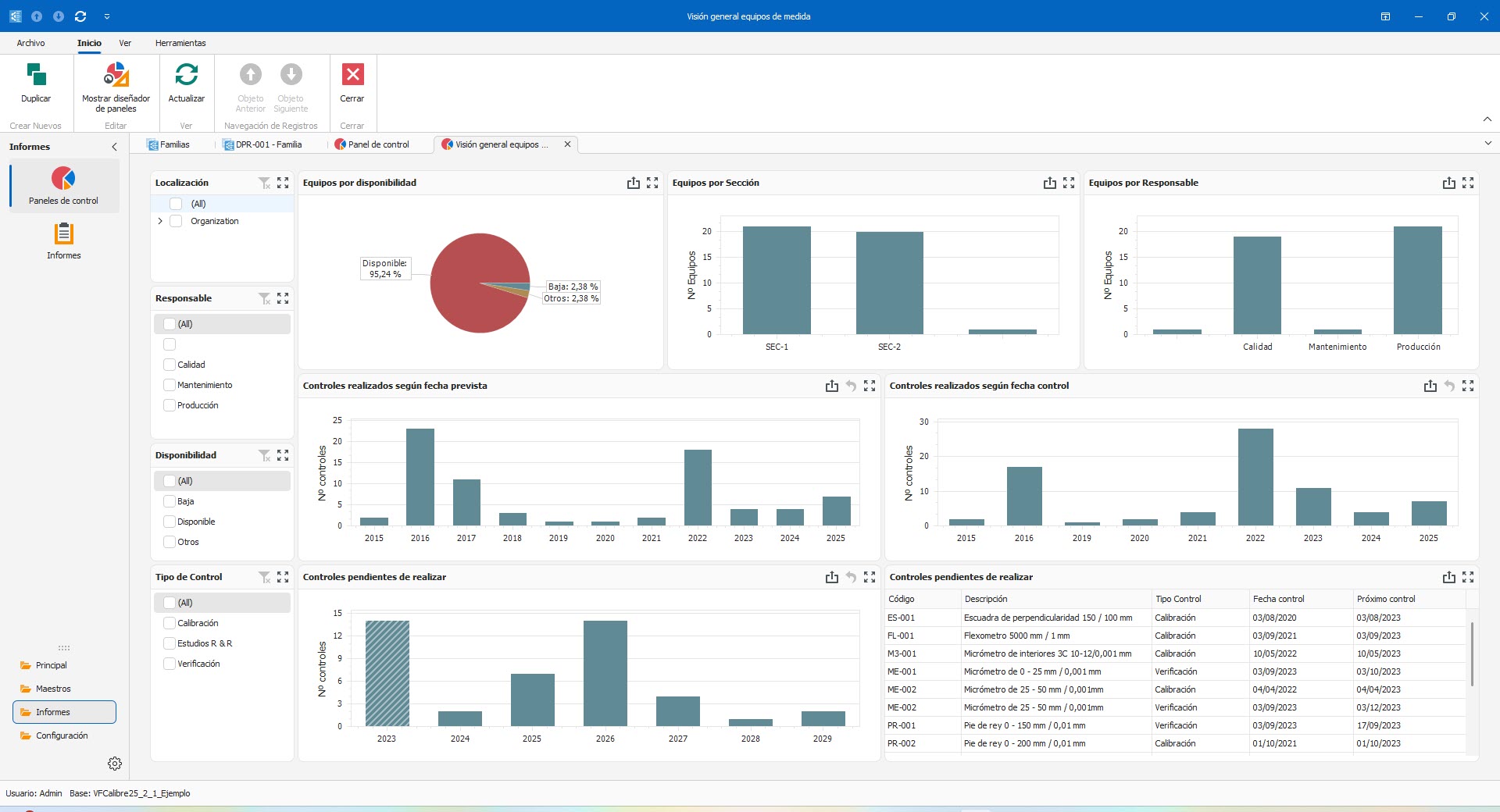
- Comprehensive dashboard for defining KPIs and monitoring objectives
- Quickly obtain predefined reports, certificates and labels.
Visual Factory Calibre is the application you need in your metrology laboratory.
Benefits of our calibration software
Easy to install and use
References used
Visual Factory Calibre Editions
Visual Factory Calibre SME
Ideal for small and medium-sized companies with a small fleet of measuring equipment. Designed to use external and internal calibrations with standard calibration or verification processes included in the application itself.
Visual Factory Calibre Profesional
Designed for companies with a larger volume of equipment and where multiple metrology technicians need simultaneous access. This edition includes specific features such as data export to different formats, import from other Visual Factory Calibre databases, and the ability to incorporate additional modules.
Visual Factory Calibre FDA
With this edition, pharmaceutical companies will be able to meet the requirements specified in FDA 21 CFR Part 11. It is also ideal for laboratories or companies seeking ISO 17025 accreditation, or simply for organizations that require greater data security, auditing of entered data, approval cycle management, and electronic signature.
Visual Factory Calibre Web Viewer
This application operates in a web environment, so information can be viewed from a PC, tablet, or smartphone. Only one of the Equipment, Equipment Controls, and Results modules can be accessed in read-only mode. Licensing is per user and is offered in packages of 50 users at a very affordable price. It is necessary to have at least a Professional or FDA license
Differences between options
| Functionality | Viewer | SME | Professor | FDA |
|---|---|---|---|---|
| Viewing application logs | ||||
| Editing application logs | ||||
| Performing controls with standard processes | ||||
| Performing controls with previously defined MS Excel formats | ||||
| MS Excel format configuration | ||||
| Report and Label Designer | ||||
| Printing reports and labels | ||||
| Dashboards | ||||
| Sending reports by e-mail and export to PDF | ||||
| Specific long-term weekly/monthly planning report | ||||
| Integrated security with Active Directory | ||||
| Import between databases | ||||
| Export to PDF, MS Excel, MS Word, … from views | ||||
| Simultaneous access from several stations | ||||
| MS SQL Server Databases | ||||
| Basic trail audit | ||||
| Audit trail with the possibility of electronic signature | ||||
| Approval cycle with electronic signature | ||||
| Definition of time window for performing the calibration | ||||
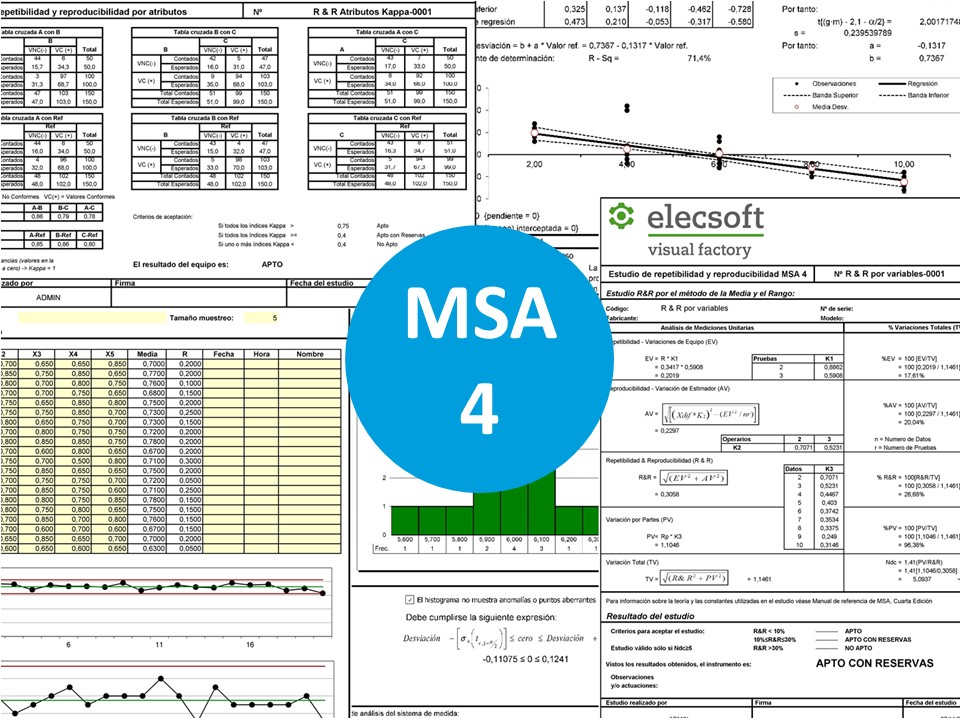
| MSA 4. R & R for variables (Mean and Range Method) | ||||
| Full MSA (includes AIAG MSA 4) | ||||
| Traceability between teams | ||||
| Calibration history | ||||
| Linked files and/or attachments | ||||
| Presentation of images in the family and in the team file | ||||
| Customizing views public and private to filter, group, sort and format the presented records | ||||
| Viewing public and private views | ||||
| Field customization and literal |
Visual Factory Calibre can be extended with the following modules:
|
Department and Location Access Module The department and location access functionality allows us to restrict or filter the equipment, equipment controls, results, and applications that the user can consult or access based on the organizations, sections, or departments to which they have access/assignment. |
|
Input and Output Management Module The objective of this module is to facilitate the input and output of equipment in the laboratory, as well as the dispatch and reception to external laboratories. |
|
Input Registration Module Locations The location registration system developed to work with Visual Factory Calibre allows you to use a standalone program, Location Registration, from which you can make location changes. It also allows you to use it directly from Visual Factory Calibre using the Location Registration module. |
|
Usage Control Module In addition to periodic monitoring, this module allows you to define equipment monitoring based on its usage. This module is closely related to the Location Registration module, which tells you whether a piece of equipment is being used or in storage. It is also possible to obtain information about whether or not a piece of equipment is being used through integration with automated warehouses. |
|
Email Notifications Module This module allows the administrator to configure notifications for the users responsible for executing the controls. The application will systematically check when calibrations and/or verifications or other controls are to be performed. Pending calibrations will be emailed to each user responsible for performing the controls in the corresponding Section or Department. The administrator can set the frequency, expiration date, and the list of users allowed to access the information. |
|
Control Approval Cycle Module Allows you to establish a workflow for performing controls on measuring equipment. This allows you to edit, review, and approve a calibration, verification, etc. This module is only available for professional editions, as the FDA already includes this module. |
|
PDF Certificate Generation Module Automatically generates and stores a copy of the completed certificate in PDF format. The objectives of this module are as follows:
|
|
Digital Signature Module Allows you to sign PDF certificates with digital signatures and validate them with certificates issued by a Certification Authority (CA). |
|
Extended FDA Audit Trail Module This module allows you to:
|
|
Equipment, Instrument, and Loop Management Module Allows you to associate an equipment record with different concepts associated with the equipment type. |

![Captura de la sección “Incertidumbre” de la aplicación Visual Factory Calibre, donde se muestra: Título “INCERTIDUMBRE” y la ecuación principal Y = y + Corrección ± U. Texto explicativo “Incertidumbre típica combinada (Magnitudes de entrada no correlacionadas):” seguido de la fórmula uᶜ²(y) = Σᵢ₌₁ⁿ [cᵢ·u(xᵢ)]² = Σᵢ₌₁ⁿ uᵢ²(y). Dos paneles azules con los métodos de cálculo que Visual Factory Calibre realiza automáticamente: Evaluación tipo A: u(x̄) = (1/√n)·√[Σᵢ₌₁ᴺ (x̄ – xᵢ)²/(n–1)] (Repetibilidad – Regresión lineal – Deriva) Evaluación tipo B: • Patrón: u(x) = Uₚ/kₚ • Distribución rectangular: diagrama con LI y LS y fórmula u(x) = (LS – LI)/√12 (Resolución – Histéresis – Excentricidad) Al pie, “Incertidumbre expandida: U = k·uᶜ”, indicando que Calibre calcula tanto la incertidumbre típica como la expandida.](https://www.elecsoft.com/wp-content/uploads/2025/03/Incertidumbre.jpg)

![Captura de pantalla de la interfaz de la aplicación Visual Factory Calibre, en la sección de “Familias” para el ítem “DPR-001 – Familia – Pies de rey Ø 0 - 150 mm”. La ventana muestra: Cinta de opciones con pestañas: Archivo, Inicio, Ver, Herramientas, Edición (activa), Insertar. Botones de portapapeles (Deshacer, Rehacer, Pegar, Pegado especial). Barra de navegación lateral con iconos: Familias (seleccionado), Equipos, Formatos, Controles Equipos, Reportes, etc. En la zona principal, pestañas: Datos generales, Posibilidades de medida, Controles, Configuración avanzada (activa), Archivos vinculados, Archivos adjuntos, Audit Trail. A su derecha, un panel con la vista previa de un calibrador (“pié de rey”). En el área de documento (configuración avanzada), texto de sección “5.5.- CÁLCULOS” con la explicación de la incertidumbre típica en cada punto de medida: Ecuación u² = u₀² + uₛ² + uᵣ² Definiciones: u₀ = I₀/k₀ (incertidumbre tipo B por patrón) uₛ = S/√n (incertidumbre tipo A por repetibilidad) uᵣ = R/√12 (incertidumbre tipo B por división de escala) S = √[Σ(x̄ – xᵢ)²/(n–1)] (desviación típica) Pie de ventana indica “Usuario: Admin Base: VFCalibre23_0_8_Ejemplo”.](https://www.elecsoft.com/wp-content/uploads/2025/03/VFC23_Familia_PR.jpg)